DHF - Design History File
In the earliest stages of developing a new medical device, especially from an entrepreneurial perspective, when we think FDA, it is often in the context of “Pathway to Approval.” Sure, we may realize that one day we will have a medical device company with a quality system in place. We may realize that we’ll have to someday be following Good Manufacturing Practices (GMP). And we are most certainly thinking about how we are going to get an FDA clearance or approval to market the new device. These are all looming in the future. It may be a surprise for some inventors and innovators that the design process itself is part of these regulations. They are impacting you now. They theoretically started yesterday.
An experienced medical device company well aware of design controls may have a different perspective but similar result. The idea of implementing them too early can seemingly constrain the project from the free form exploration that may be best suited outside the bounds of a “controlled” design process.
According to the FDA, the design history file shall contain or reference the records necessary to demonstrate that the design was developed in accordance with the approved design plan and the requirements of this part (21 CFR Part 820.30). Each manufacturer shall establish and maintain a DHF for each type of device.
It is this DHF that is the primary tool for ensuring the medical device was designed according to 21 CFR 820. If following ISO 13485 (recommended), the DHF then demonstrates design process is in accordance with that standard. The takeaway here is that a DHF is the key set of documents demonstrating the design of a medical device was controlled according to some standard.
Design History File-Ready Ideation is a set of tools that encourage creativity best practices while at the same time building the foundation of design controls. It is DHF “ready” because it is intended to be used long before the formal implementation of the QSR. It can also be used as a creative brief to build presentations and pitch decks. And it can continue on after crossing the dividing line into a controlled process, to persist the creative mindset throughout the development lifecycle.
Virtual Whiteboard
Update: as of 3/24/2020 the DHFRI Training Canvas has been updated to Version 3. This includes two new canvases, which include the Stakeholder Journey Canvas and the Research and Design Canvas.
To explore the training canvas, please enter anonymously.

So what is DHF Ready Ideation?
It is a tool set ultimately realized in the form of canvases.
What are canvas tools?
My first experience working with a canvas tool was with Business Model Canvas. It was originally developed by Alexander Osterwalder in his book, Business Model Generation, and has since served as a useful approach to visually capture business strategy. These types of visual frameworks have been around forever. Methods such as empathy maps, roadmaps, and mind maps are all examples of such visual design aids. The genius of Business Model Canvas is the context that it’s able to capture and lends well to exercise and facilitation. What’s particularly nice about these tools are the ways in which they can be applied in individual and/or group settings. Canvases can be printed on large format paper and placed on a boardroom table, sketched on a large post-it note and placed on the wall, drawn out on a whiteboard, fit on a single page in an illustrated book or from a desktop printer.
One particularly useful implementation is via the use of digital whiteboards. There are multiple benefits to these web based tools including:
- Enabling both individual and group sessions
- Providing richer content beyond notes and sketches, such as hyperlinks to resources and images grabbed directly from the web
- Memorializing progress over time
There’s no need to take anything down and transcribe it when your whiteboard is ever present in the cloud, whether living in a state of continuous improvement or frozen for historical reference.
Elements of DHF Ready Ideation
DHF Ready Ideation is implemented in the form of four such canvas tools, including The Diligence Dashboard, reimagined Waterfall, Human Factors Engineering via Prototyping, and Risks/Hazards. Using a digital whiteboard, all four of these tools can live together providing a holistic and particularly enlightening view to the complex multi-dependent nature of medical device product development and thus inspiring creativity.
Breaking down each canvas
Diligence Dashboard
There’s a lot of history that went into developing the Diligence Dashboard. This includes being a design engineer at a medical device company, co-founding an engineering services firm focused on medical devices, starting medical device companies, and teaching design from the context of a Biomedical Engineering professor. All that said, the crystallization of these perspectives came together from a concept known as “The Killer Experiment” as taught by The Wallace Coulter Foundation.
A little background on Coulter: Wallace H. Coulter was inventor of the Coulter Counter and entrepreneur that started the Coulter Corporation, which later was acquired to become Beckman Coulter. When Wallace passed away, he left his entire fortune to the foundation; one of two foundations (along with Whitaker) that was primarily responsible for Biomedical Engineering being an academic department at about 150 universities across the country today.
In 2005, the Foundation launched the “Early Career” (EC) program which later was rebranded as the “Coulter Translational Research Award” (CTRA). These grants promoted collaboration (it was a requirement) between an Academic Researcher and a Medical Provider; and included commercialization training to the team. During this training, the Foundation and its business advisors coined the term "Killer Experiment.”
What exactly did the faculty of Coulter mean by this Killer Experiment? Often times academics are in search of an area of research that they can devote their careers to. This devotion leads to a thought process that works by first having a hypothesis, and then considering experiments to support that hypothesis. Sometimes they are supported, sometimes they are not; In both cases, learning happens. They then may propose a new hypothesis, and consider experiments to support that one, and the cycle continues.
Sure, there are incredible success stories and outcomes of this research changing our world. However, often times, a researcher can spend their life on an investigation that never reaches the market. Coulter was committed to getting products to the market such that they could have real impact on patients’ lives.
The Killer Experiment was intended to change the mindset of academics to asking a different question: “What is the next experiment I can do that would kill my research?” This is not exactly intuitive when you are a young investigator in pursuit of tenure. Coulter was rewarding such young academics by providing these grants, and promoting a commercial mindset.
But why promote this mindset? Because time is the most valuable resource. There’s an infinite well of problems to be solved. Where there’s a valuable solution, there’s money to be found. Time, however, is a resource that can never be replenished. It was Coulter’s way of saying: spend your time on projects that will change the world.
Over the years, the business advisors to Coulter brought a new spin to the Killer Experiment and started calling it “The Quick Kill Concept.” This is because, sometimes, the thing that can derail a project from success is not always a technical failure, but a business hurdle. In Medical Devices, this is a multi-body problem that includes factors such as regulatory burden, market size, reimbursement, technical complexity, and intellectual property position.
It is worth noting that the words “Killer” and “Kill” can sometimes turn off a researcher or investigator because they seem so final. They don’t mean, necessarily, to “quit” or "end" or "stop" a project. I prefer to think of this as a tactical strategy. There’s many obstacles, and they come at you over and over again… one technical hurdle after another and another…, a business hurdle, and another and another. In the worst case, without this foresight, you may work for years and get unexpectedly hit by a Mack Truck. With the Quick Kill mindset, sometimes you may choose to quickly kill the project. Sometimes you choose to pivot. At least you know the truck is coming.
All that being said, I’ll offer another evolution from “The Killer Experiment” to “The Quick Kill Concept” to “Diligence Dashboard.”
So, what's this Diligence Dashboard all about anyway? The product development process in the medical device industry is inherently stage-gate due to a requirement for design reviews. Product development cycles can take years progressing through stages that are marketing heavy in the beginning, design and engineering focused in the middle, transitioning to manufacturing, then regulatory approval, and near the end, establishing reimbursement. Such an investment of time typically means that intellectual property claims be strong and there's a large market waiting at the finish line.
All too often, we see medical innovations with millions invested, but worse, years spent, before a later stage factor, such as reimbursement, kills the project. What if we could have predicted that failure before we started?
The Diligence Dashboard provides intelligence at the fuzzy front end of a project that can provide insights to potential failures, go-no-go decisions, and/or pivots to increase the chances of success for projects you're investing time and money in.
Realized in its canvas format, the Diligence Dashboard visually lays out five areas that the Coulter team taught as part of its commercialization process. These include technology feasibility, market size, intellectual property, regulatory pathway to approval, and medical economics (including reimbursement).
The Waterfall Canvas
The waterfall design process became famous when it was presented as part of the FDA’s Design Control Guidance for Medical Device Manufacturers published on March 11, 1997. The figure, which was used by permission of the Medical Devices Bureau, Health Canada, has been pervasive in the industry, more or less defining they way in which design engineers performed their jobs. The fine print in the guidance document, just below the figure’s reference, states, “In practice, feedback paths would be required between each phase phase of the process and previous phases, representing the iterative nature of product development.” Standing alone, without this fine print, the figure provides little indication of iteration, and is primarily interpreted as a traditional waterfall that follows a logical sequence of phases.
If the downside of waterfall is its apparent missing components of iteration, its upside is the focus on user needs, regular review, and success criteria based on verification and validation. The intent of the reimagined Waterfall Canvas is to retain the benefits while combating pitfalls of a stage-gated interpretation. Note: the FDA’s reference to waterfall was never meant to be a prescription for development. Iterative methods, including agile, are acceptable (and may even be preferred) within the bounds of design controls.
When the waterfall process is implemented as a linear stage-gate, it is easy to imagine a scenario where user needs (first step) are gathered prior to formalizing design controls. Progress “seemingly” worth documenting, especially in an entrepreneurial venture, may not be gained until the 4th step (design output). It may take years to get there. It is precisely this first step (user needs) and resulting design inputs that can be so cost prohibitive to go back and recreate after the fact.
The Waterfall Canvas is a new way to look at the process that highlights the originally intended iterative nature of design, while capturing those needs along the entire evolution of design (starting well prior to implementing a quality system). The canvas provides the users a place to capture raw, unfiltered research (such as interviews and observations) to feed the process like a flywheel.
In the classic layout of the waterfall process, verification of design outputs maps back to design inputs. Validation of the medical device maps back to user needs. However, the “Design Process” stage is often described as a black box where design happens. It is left out of an explicit mapping. In contrast, the canvas format redefines this space as “Design Concepts” and is provided with the largest swath of space on the board.
Human Factors Engineering (HFE) Prototyping Canvas
The focus for HFE in medical device product development first appeared in 1996 when they were added to the FDA’s Quality System Regulation. The 20+ year history of including HFE into the regulatory framework includes a number of references, included as an appendix to this guidebook.
Today, awareness of HFE is increasingly growing as regulatory submissions get rejected by the FDA and other regulatory agencies around the world. The bottom line is that HFE must be rolled into the overall design control process and integrated into risk management efforts. This is a topic that can no longer be ignored, and is therefore an integral part of the DHF Ready Ideation suite of canvases.
At a minimum, the HFE Prototyping Canvas is a tool to encourage the use of multiple prototypes in an effort to inform design direction. In the context of a holistic view of DHF Ready Ideation, this board works hand in hand with the Risks and Hazards Canvas (discussed below) and the Waterfall Canvas to visualize the iterative and formative process of design. Deeper discussions on prototyping and on HFE are included a little later in this guidebook.
Risks and Hazards Canvas
Critical to any medical device product development process is the consideration of potential harms. There’s a seemingly infinite number of methods to assess risks and analyze hazards. During my time as a product development engineer and later, biomedical engineering professor, the tool of choice was Failure Modes Effects and Criticality Analysis (FMECA). However, FMECA doesn’t lend itself well to a visual and creative modality, thus, I chose to implement a matrix model in DHF Ready Ideation.
Key to implementing the Risks and Hazards Canvas are the following definitions:
- Harm: a negative event such as illness, injury, or death
- Risk: the probability of a harm occurring
- Hazard: the possibility of causing harm
- Exposure: the action that leads to the harm
Referring to the canvas itself, you’ll see that the horizontal labels across the bottom represent probability of risks ranging from highly unlikely to highly likely. The vertical labels up the left side of the matrix represent severity of harms ranging from no impact (not even a scratch) to extreme severity (death or dismemberment). The hazard analysis can then be performed visually by brainstorming exposures and plotting them with respect to probably of risks and severity of harms. The combination of the severity and probability is the resulting risk of harm and is represented by where the two intersect on the grid.
As a quick example, imagine the risk and hazard associated with plugging in a charger for your electric toothbrush. The outlet is a hazard. It’s always there, whether you interact with it or not. The harm is getting electrocuted by the action (exposure) of plugging in the power cord while your wet hands are touching the prongs. The risk is the probability of that harm occurring.
In this example, the risk can be lowered by education (instructions for use by the manufacturer, but, more likely good parenting), or better yet, by product design (a grip on the power cord that minimizes likelihood of touching the prongs). The harm can be reduced by adding a ground-fault circuit interrupter (GFCI) to all bathroom outlets.
A Deeper Discussion on Prototyping
Here’s an all too often scenario: An inventor is working for months, maybe years, to prove a concept on the bench. Let’s say it’s an invention for a new medical procedure. After getting the functional proof of concept working on the bench, they’ll spend another several months getting that prototype into a form presentable to stakeholders. The doctors, nurses, and other caregivers they show the prototype to respond with similar answers such as, “I’ll never use that,” and “it’ll never work.”
Digging a little deeper into the logic behind their responses, we find it’s due to the prototype’s inability to be sterilized, or it’s not suitable for cleaning protocols, or it’s too big and heavy for their use case. But maybe that feedback had nothing to do with why the inventor wanted to meet with and talk to stakeholders to begin with.
What was it they were looking for?
Let’s start with “Why” we’re building a prototype. Do we have a basic science question we are exploring? Is it perhaps an engineering question we want to answer with the prototype? Are we looking to show a product to a potential investor or company decision maker? Are we seeking feedback from a user? Do we want to see how a product feels during everyday use? How it fits into a physical space? Are we curious as to how consumers may react to a brand asset?
These are all very different reasons to build a prototype, and they all require different prototypes. I like to break down prototypes into four categories of why we want to build it: Form, Function, Fit, and Feel.
Form - A “looks-like” prototype to attain feedback with respect to aesthetics.
Function - A “works-like” prototype to attain feedback with respect to user experience and functionality.
Fit - A prototype that demonstrates how multiple components are assembled or how a product fits within an environment.
Feel - A prototype primarily designed to attain ergonomic feedback.
I like these four categories, but these are not the only ways to define “prototype.” From these definitions alone, it can be seen that prototypes can be broken down into even more refined groupings. Prototyping is an extremely broad term and can include prototyping versions of code for a software implementation, prototyping business models to inform launch strategy, and a myriad of other such examples.
With that in mind, this aside will stay focused on the scope of product and brand assets. (Which, when done well, are the same thing. Think Coca-Cola bottle.)
“At Trig, we consider a prototype to be any demonstration of a product and/or brand concept prior to a commercial product and/or published marketing asset.”
— Trig Team Definition
In addition to the desired feedback (Form, Function, Fit, Feel); there are additional dimensions to consider including:
Resolution (aka Fidelity): Low to High
This is used to determine how refined the prototype is. If a physical model, low resolution may be made from Legos, while high resolution can be manufactured using 3D printing technology.
Dimension: 2D vs 3D
Paper and/or digital images versus physical models.
Cost: Low to High
From a few cents on the dollar to thousands of dollars.
Speed: Real time to Weeks
Exactly what it sounds like.
Once we know “Why” we want to build a prototype, or multiple types of prototypes, let’s consider “What” kinds of prototypes there are. Between Feedback, Resolution, Dimension, Cost, and Speed there are a seemingly infinite number of options. Here are some of the more common types of prototypes we may see, just as a few examples:
Sketches & Diagrams
These can be from rough to refined, done in real time, or crafted over time, and used for capturing concepts and communicating concept direction.
Renders (aka Renderings)
Some renders can be built using Computer Aided Design software, such as SolidWorks, and may focus on surface features for fit and manufacturing. Others may be photorealistic renders with a focus on marketing quality images often shown in context.
Wireframes
These prototype a digital experience and are typically static images that demonstrate website, app, or other embedded software user interfaces.
Storyboards
This is a type of drawn prototype which illustrates not just an item, but interactions with the item. Storyboarding gives designers an opportunity to showcase scenarios in which their invention will be used and what the user experience will be like.
Role Playing
Much like storyboarding, role playing focuses on scenes to put an item into real world context. Rather than a sketch, you act the scene out in real life, often with partners to assist, take notes, or play different roles.
Physical Models
Create a version of the design using materials that are easy to obtain and easy to rework. Build a model and test various usability factors. Physical prototypes can be created out of more things than you may realize. Get creative.
We often think of prototypes as 3D models. Depending on your “Why” - these could range from tools like Legos, Play-Doh, and pipe cleaners to foam models to rapid manufacturing techniques such as laser cutting and additive/layered manufacturing (aka 3D printing) including SLA, SLS, FDM, etc. The bottom line: there are a myriad of low-tech and high-tech methods to choose from.
The 2D forms of prototyping can be as simple as a Pen and Paper and can include more complex tools such as CAD and particular programs including SolidWorks, Keyshot, Photoshop, and Illustrator to name a few.
Sometimes we don’t need to make anything and can use existing items such as props to create the prototype.
What is the context of your current situation? Maybe you are an inventor with a concept and your goal is to raise some seed funding. Maybe you are a product development engineer at a medical device company and your goal is to gain formative feedback to meet your HFE regulatory requirements. Whatever the situation, consider that your goal may be harder to achieve with one very complex prototype, as opposed to thinking rapid, iterative, affordable prototypes (multiple different prototypes) that are context specific.
It’s like using triangulation as a method to determine the position of a target. Rather than using one complex system, from one point of view, the use of three lower technology devices, from 3 or more positions will provide more meaningful results.
What is Human Factors Engineering?
HFE can be a complex subject, it is however also one of common sense. In my opinion, the latter is a must have, while the complexity is case dependent. HFE is referred to in the popular press and scholarly manuscripts by many names including ergonomics, human engineering, engineering psychology, and human factors psychology to name a few.
If I were to recommend any one book on the topic, it is The Design of Everyday Things, otherwise known as The Psychology of Everyday Things. This single book with two titles was written by cognitive scientist Donald Norman. My CliffsNotes version of the book in one sentence is: “It’s not user error, it’s use error.”
When a product is improperly used, or when a process is improperly followed, which in either case may possibly result in an unwanted outcome; it is often the user that takes the blame. Norman’s book is a master class in psychology and design that puts the spotlight on the product or process itself as the culprit.
In medical devices, the unwanted outcome from such improper use can result in harm to the user. In any commercial product, at a minimum, unwanted outcomes impact user satisfaction and ultimately sales of the product. HFE is not just a must mandated by regulatory bodies, it is a smart business decision.
The bottom line: the product itself should promote proper use and result in desired outcomes. Nobody gets hurt and it’s a joy to use.
HFE considers all the ways a user interacts with a product. This might include cognitive factors, such as memory capacity, multitasking and reaction time. It involves product design features that may inform how an item is used, such as colors and other indications, for example impressions that indicate hand placement. It takes into account ergonomic considerations such as variations in body size, range of motion, and strength.
Some of the key activities involved specific to medical device product development (as part of the standards and regulations) include defining intended users and use environments. HFE should be an integral part of both the design process and when assessing risks and hazards, thus used to both improve design and minimize potential harms.
As an example, the user of a medical device may be the patient that it’s being used on, the doctor that’s using it, and any other medical professional, such as a nurse, that may be interacting with it. In the toothbrush example used above, one user may be a child that’s brushing their teeth and another user a parent that’s maintaining the product (such as replacing brush heads). The use environment is a bathroom, typically a place that is associated with water (sinks, tubs, toilet bowls).
In design, we may refer to the different user groups as personas, and capture information such as occupation, demographics, and experience level as a few examples. The use environment can include features such as layout, furniture, equipment, lighting, noise, additional people and pets in the environment, and any other potential distractions.
Stakeholder Journey Canvas
New for Version 4, we are introducing the Stakeholder Journey Canvas. Along the Y-axis, we list the 3-5 stakeholder groups who might engage with the product during it’s active use life cycle. Along the X-axis, we list the specific steps required to complete the job for which the product has been hired.
A suggestion for the stakeholder journey steps might be to reference the 2008 HBR article, “The Customer-Centered Innovation Map” by Lance Bettencourt and Tony Ulwick. The authors claim that there is a universal structure to every Job-To-Be-Done with eight categories of job steps called a Universal Job Map. While a Stakeholder Journey isn’t the same as a Job Map, the steps as described are a useful starting point for understanding your Stakeholders. As listed below, we have changed some of the descriptors to apply to the healthcare industry:
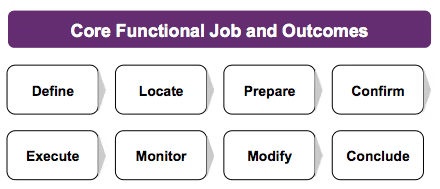
- Define: (Plan, Select, Determine) stakeholders determine their goals and plan resources.
- Locate: (Gather, Access, Retrieve) stakeholders gather items and information needed to perform the procedure.
- Prepare: (Set up, Organize, Examine) stakeholders set up the environment to perform the procedure.
- Confirm: (Validate, Prioritize, Decide) stakeholders verify that they are ready to perform the procedure.
- Execute: (Perform, Transact, Administer) stakeholders carry out the procedure.
- Monitor: (Verify, Track, Check) stakeholders observe the results to confirm it was performed correctly.
- Modify: (Update, Adjust, Maintain) stakeholders make modifications to improve how the procedure was executed.
- Conclude: (Store, Finish, Close) stakeholders finish the procedure or prepare to repeat it.
For example, if the desired outcome is “connect with family during COVID-19” then the jobs may look like this:
- Define
- Define who to connect with
- Define how to connect (phone, video, email, snail mail)
- Locate (what I need to connect with family)
- Could be pen, stationary, stamps
- Could be Zoom account, computer with video
- Prepare (for the connection)
- Could be: Find a quite and private place to talk
- Confirm
- Could be to double check that you have everything in order to make the connection (eg. Make sure the oven is off before I walk outside to make a call)
- Could be to confirm that the other person is available at the desired time of connection
- Execute
- This is when you make the call, write the letter, etc.
- Monitor
- Take notes. “I liked Zoom, but, next time I want to do it differently.”
- Or: “Outside calls are noisier than I thought with this wind.”
- Modify
- Go inside to escape the wind
- Go back to “Locate” a new pen, because this one ran out of ink
- Conclude
- Drop the letter in the mailbox or say goodbye before hanging up
This is a simplified version of the job steps to complete a personal connection when social distancing. In practice, we would drill down on each of these job steps and brainstorm all the ways one might Define, Locate, Prepare, etc. all the jobs to complete a desired outcome. As the desired outcome becomes more complex, so does the complexity of the associated jobs.
Another suggestion for the stakeholder journey steps might be to use the Hero’s Journey as described by Joseph Campbell in the 1949 book, The Hero With a Thousand Faces. As an archetypal narrative structure, we respond emotionally to the explorer’s journey out into the unknown to brave danger and bring back valuable treasures to benefit society.
- Call to Adventure
- Meet a Mentor
- Trials and Failure
- Growth and New Skills
- Death and Rebirth
- Revelation
- Atonement
- Receives Gifts
- Returns Changed
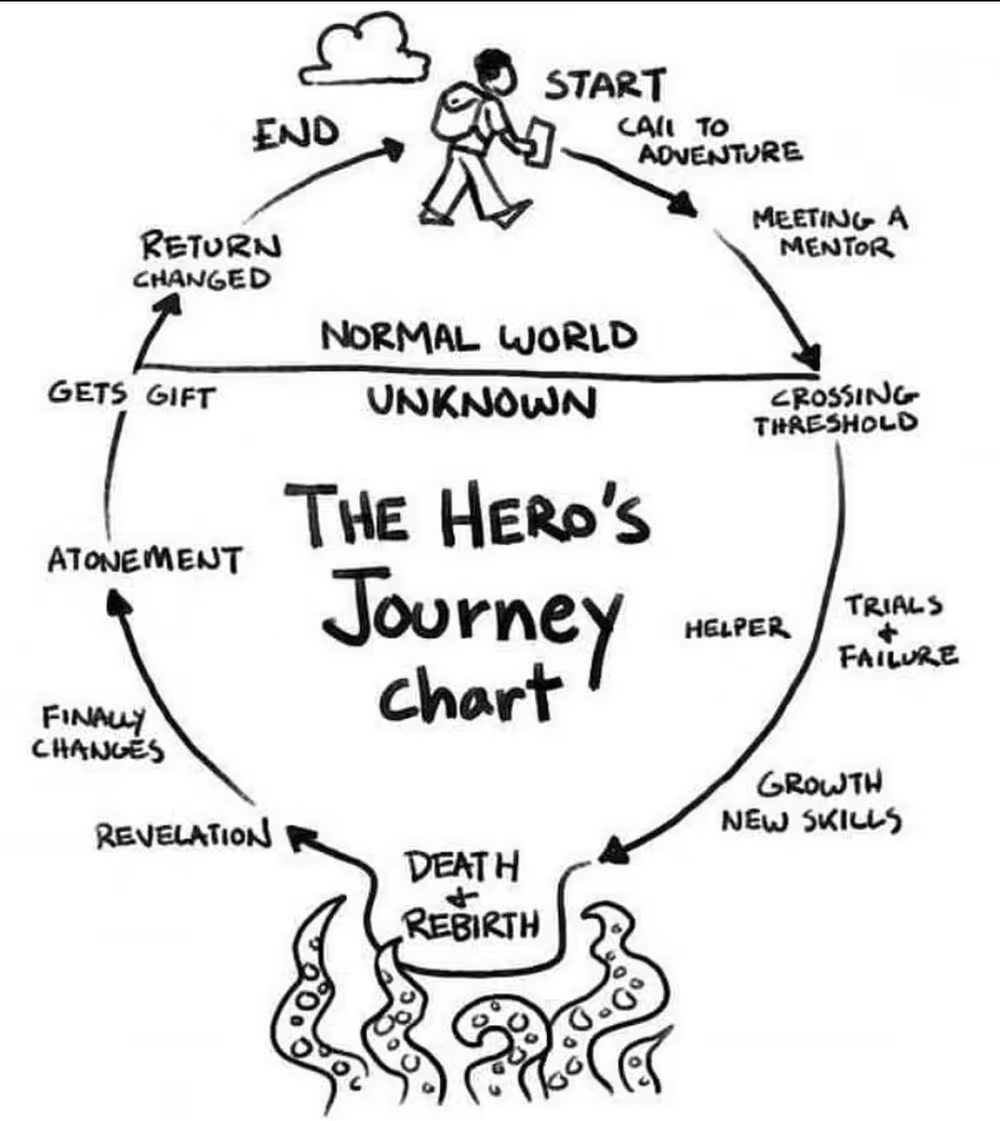
The Hero’s Journey is particularly useful for describing the training process among multiple stakeholders - trainees, trainers, and support team. The scope of the Hero’s Journey is more broad and emotion-driven than the Universal Job Map, which is more narrowly defined and logic-driven. Both are helpful suggestions to start mapping the specific journey of the stakeholders for whom you are seeking to understand.
Bringing it All Together
Medical device product development is a complex process that includes business and technical risks in a highly regulated and political environment. The DHF Ready Ideation canvases bring together a holistic view of this environment to promote an iterative process even before starting formal design controls, such as those required by the FDA. This tool is intended to improve commercial success, reduce use error and thus minimize harm to users, and provide traceability while encouraging iterative design.
How can a tool like this establish traceability prior to implementing design controls?
In practice, it is a rare exception that a project starts from a blank slate. At some point, a team comes together and begins investing time into a project that has gained momentum from some original source. Maybe it’s a skunk works project within a larger company, a lead coming in from marketing, a medical doctor with an innovation, or a researcher that has invented a new technology.
Whatever the case, imagine deploying DHF Ready Ideation at a given point in time based on previous work. This first canvas is the initial conceptual understanding of the project from a holistic perspective looking at business risks, hazards, and human factors. There’s likely to be considerable gaps.
Best practice implementation of DHF Ready Ideation includes date stamping this starting point. Over time, through multiple iterations of the waterfall, risks/hazards, HFE prototyping, and diligence dashboard feeding each other, an inflection point will occur. Maybe it’s a significant design direction. At this point in the project, freeze the board and date stamp it again.
A copy of the frozen board becomes the starting point for a new one. Delete all concepts no longer relevant and their associated dependencies across the board. Keep the concepts still in play, and date stamp the start of a new canvas. Over time, a series of frozen canvas tools will demonstrate traceability, even before working within a quality system.
That first day of design controls begins with a mature view of the project, with well written needs, inputs, and outputs based on formative research and risk reduction over time.
